Greetings from the representative
 ShinAkasaka Clinical Laboratory
ShinAkasaka Clinical LaboratoryCEO Ryosuke Minakata
We will provide swift and high-quality clinical trial implementation.
The Drug Discovery Division of the New Akasaka Clinical Testing Center was established in 1983 as a Site Management Organization (SMO) to support clinical trials in medical institutions. Serving as a bridge between medical institutions, patients, and pharmaceutical companies, we prioritize the importance of human connections and provide high-quality operational support. Our distinctive feature lies in our focus on supporting clinical trials, particularly in the field of ophthalmology, and investing in the education of trial coordinators, enabling us to offer efficient and high-quality support for clinical trials. While our drug discovery division celebrates 40 years of independence, the industry landscape is constantly evolving. Leveraging our strength in the field of ophthalmology, we continue to adapt to changes and expand our capabilities to accommodate other therapeutic areas, striving to contribute further to the field of drug discovery.
| Name | ShinAkasaka Clinical Laboratory |
|---|---|
| Line of Business | SMO (site management organization)) |
| Address | 4th floor, FORECAST Ningyocho PLACE 3-4-14 Ningyocho, Nihonbashi, Chuo Ward, Tokyo, 103-0013, JAPAN |
| TEL/FAX | TEL:03-3662-0031 / FAX:03-3662-0033 |
| Established | November 1969 |
| Representative Director | Ryosuke Minakata |
| Director | Ryoka Sunatori |
| Number of Employees | 29 |
| Member Organizations | Strategic Management and Operation Network Association for Clinical Study (SMONA) Japan Society of Quality Assurance |
| Clients | Major pharmaceutical companies throughout Japan, among others |
| Banks | Mizuho Bank MUFG Bank Kiraboshi Bank |
Information
-
2023.03.20
This website has been updated
-
2022.04.04
This website has been updated
-
2021.11.02
We have launched the English version of the website
-
2021.02.01
Yosuke Minakata has assumed the position of President and CEO
-
2020.04.08
Notice of Implementation of Telework due to the Declaration of State of Emergency
-
2020.03.13
Our Measures Against the Novel Coronavirus (COVID-19)
-
2018.10.18
Workshop on Interpretation of Clinical Test Values by Professor Kazuo Hatamura from the Faculty of Medical and Health Sciences, Department of Medical Laboratory Science, Kitasato University
-
2017.09.11
We have relocated our business office
ShinAkasaka Clinical Laboratory
To all healthcare institutions
Clinical Trial Support Services Ensuring Safety and Trust
Limited Liability Company New Akasaka Clinical Testing Center, in its role as a Site Management Organization (SMO), primarily provides support for clinical trial operations conducted in medical institutions. In detail, we assist in outsourcing pharmaceutical development projects, particularly Phase II trials (exploratory trials), Phase III trials (confirmatory trials), Phase IV trials, and others from pharmaceutical-related companies. We support the entire process, from introducing clinical trials to medical institutions to their completion.
Experienced and Knowledgeable Clinical Research Coordinator (CRC)
CRC acquire the necessary specialized knowledge and skills for their tasks through various training curricula. Through extensive experience, they strive to improve the quality of their work by fostering effective communication at all times.
The Japanese Society of Clinical Pharmacology
and Therapeutics Board-certified CRCs
11
SMONA-certified CRCs
26
Healthcare Professionals with Qualifications
(Clinical Laboratory Technicians, etc.)
**

Thorough Support for Other Administrative Tasks
By providing comprehensive support for the intricate tasks of clinical trials, our company helps alleviate the burden on attending physicians and staff, leading to an enhancement in the quality of the trials. Additionally, we are pleased to introduce new clinical trials.
- The Introduction of Clinical Trials
- Information Provision and Investigation
- Trial Preparation
- -Assistance in Creating Documentation for Clinical Trial Review
- -Contract Negotiation Operations
- -Standard Operating Procedure (SOP)
- Support in Creation
- Clinical Trial Office Operations
- Assistance in Medical Accounting Operations
- Subject Handling
- Management of Clinic Visits Schedule, etc.
- Assistance in Creating Documentation for the Institutional Review Board (IRB)
- Creation, Storage, and Management of Essential Documents
- Responding to Clinical Trial Sponsors
- Handling Audits and Regulatory Authority Investigations
The Process of Clinical Trials
By conducting clinical trials, we contribute to the improvement and advancement of healthcare. As a medical institution capable of conducting clinical trials, we can instill a sense of trust. Additionally, we have the ongoing opportunity to acquire information on cutting-edge drugs and treatment methods, and anticipate research income.

Pharmaceutical Company
(Sponsor of the Clinical Trial)
(CRA)
Clinical Research Associate

(Clinical Trial Investigator)
- Clinical Trial Principal Investigator
- Clinical Trial Sub-Investigator
- Other Staff
Subject (Patient)

SinAkasaka Clinical Laboratory
(SMO)
(CRC)

Pharmaceutical Company
(Sponsor of the Clinical Trial)
(CRA)
Clinical Research Associate

SinAkasaka Clinical Laboratory
(SMO)
(CRC)

Medical Institution
(Clinical Trial Investigator)
- Clinical Trial Principal Investigator
- Clinical Trial Sub-Investigator
- Other Staff
Subject (Patient)

To all pharmaceutical companies
Experience in Consignments
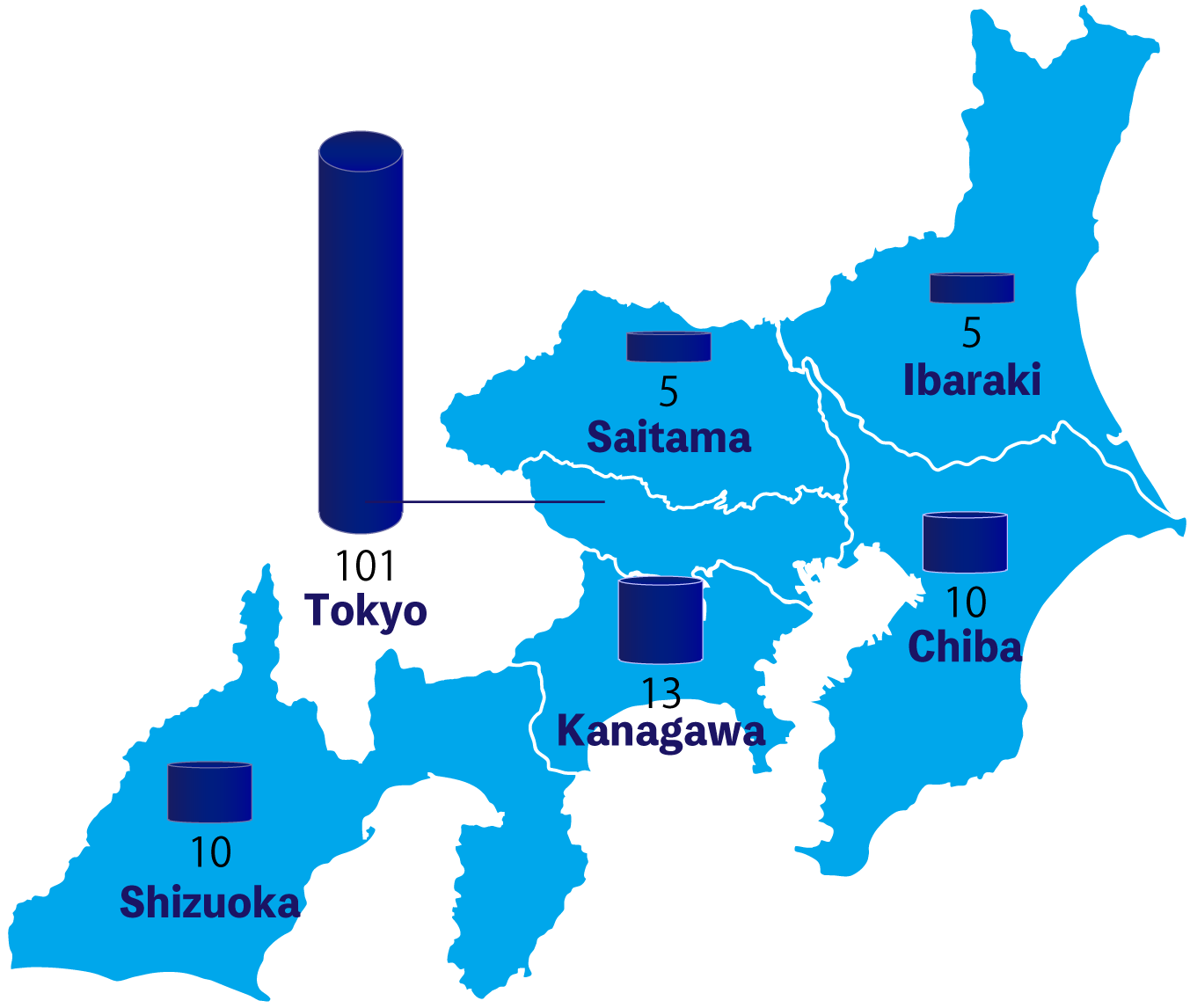
We have collaborated with over 100 medical institutions, mainly in the Tokyo metropolitan area, and have been engaged in treatment support activities. Primarily focusing on Phase II and Phase III clinical trials, we have provided extensive support for numerous trial-related tasks, especially in ophthalmology and respiratory fields. Additionally, in recent years, we have also dedicated efforts to the dermatology field.
Collaborating Medical Institutions (as of April 2023)
| University | Hospital | Clinic | |
|---|---|---|---|
| Tokyo Kanagawa Saitama Chiba |
1 | 5 | 116 |
| Ibaraki (Mito・Hitachinaka Naka-gun) |
0 | 2 | 2 |
| Shizuoka (Mishima・Sizuoka・Fukuroi・Fuji・Susono・Fujieda) |
0 | 0 | 10 |
-
114ophthalmology
-
33pulmonology
-
24internal medicine
-
12urology
-
6otorhinolaryngology
-
3dentistry
-
2dermatology
-
9other
| ophthalmology(114) | |
|---|---|
| glaucoma | 41 |
| cataract | 1 |
| dry eye syndrome | 22 |
| antibiotics | 6 |
| intraocular lens | 12 |
| anti-inflammatory drug | 1 |
| antihistamine | 3 |
| symptomatic vitreomacular adhesion | 1 |
| exudative age-related macular degeneration | 12 |
| diabetic macular edema | 3 |
| eye strain | 1 |
| pediatric myopia | 1 |
| branch retinal vein occlusion | 1 |
| central retinal vein occlusion (CRVO) | 1 |
| eyelid ptosis | 1 |
| other(diagnostic equipment) | 7 |
| pulmonology(33) | |
|---|---|
| COPD | 5 |
| antibiotic | 3 |
| antifungal medication | 3 |
| sleep apnea syndrome | 2 |
| influenza | 7 |
| pneumonia | 2 |
| asthma | 8 |
| idiopathic pulmonary fibrosis | 1 |
| chronic cough | 2 |
| chemotherapy | |
| clinical research | 26 |
| internal medicine(24) | |
|---|---|
| diabetes | 13 |
| gout | 3 |
| obesity | 1 |
| depression | 3 |
| dementia | 3 |
| RLS | 1 |
| hypertension | |
| schizophrenia | |
| gastroenterology(7) | |
|---|---|
| gastroesophageal reflux disease (GERD) | 4 |
| FD | 1 |
| chronic pancreatitis | |
| IBS | 2 |
| urology(12) | |
|---|---|
| OAB | 1 |
| interstitial cystitis | 2 |
| renal anemia | 2 |
| pruritus | 3 |
| hyperphosphatemia | 2 |
| secondary hyperparathyroidism | 1 |
| peritoneal dialysis fluid | 1 |
| otolaryngology(6) | |
|---|---|
| chronic sinusitis | 2 |
| allergic rhinitis | 2 |
| desensitization therapy | 2 |
| dentistry(3) | |
|---|---|
| dental implant | 3 |
| dermatology(2) | |
|---|---|
| onychomycosis | 2 |
| other(9) | ||
|---|---|---|
| gynecology | menopausal symptoms、dysmenorrhea | 1 |
| cardiology | drug-eluting stent | 1 |
| cancer pain | 1 | |
| catheter | 1 | |
| hematology | multiple myeloma | 2 |
| systemic AL amyloidosis | 3 | |
Delivering High-Quality CRC
We dispatch trained CRCs to affiliated medical institutions to support all aspects of clinical trial-related tasks, including trial office duties and participant interactions. Additionally, we engage in proactive awareness campaigns for trial initiation and infrastructure development to ensure smooth conduct of trials.
classroom training
On-the-Job Training(OJT)
role-playing
After completing the designated in-house training and acquiring knowledge and skills as a CRC, internal certification is awarded.
(app. 90 hrs/year)
discussion
internal study group
